
Sophie-Liliane Rosenke
Cystic Fibrosis is an autosomal recessive disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, causing the production of thick and sticky secretions into the airways. This leads to organ damage, including damage to the lungs, a major cause of early mortality. Only in the last decade, CFTR modulators were introduced, which can correct the underlying dysfunction of the CFTR gene in 90% of patients. There are currently four CFTR modulators approved in the UK: Kalydeco (the longest serving), Orkambi, Symkevi and lastly Kaftrio, introduced in 2021.
A systematic review of the literature revealed Kalydeco and Kaftrio have the greatest treatment benefit with improved lung function, reduced pulmonary exacerbations and improved symptoms overall. CFTR agents presented an overall favourable safety profile with low discontinuation rates when compared to placebo.
A greater treatment response was observed in young patients and simulations have suggested starting treatment at an early age could significantly increase life expectancy and reduce the huge life-long treatment burden of conventional symptomatic treatments. The current CFTR modulators do not have a significant effect on patients with rare CFTR mutations (10%), meaning treatment for these individuals still needs to be researched and developed.

Sophie-Liliane Rosenke
AIM
This research investigates the efficacy and tolerability of the currently available CFTR modulators which work to treat patients with CF.
Introduction
Cystic Fibrosis is an autosomal recessive disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, causing the production of thick and sticky secretions into the airways. This leads to organ damage, including damage to the lungs, a major cause of early mortality. Only in the last decade, CFTR modulators were introduced, which can correct the underlying dysfunction of the CFTR gene in 90% of patients. There are currently four CFTR modulators approved in the UK: Kalydeco (the longest serving), Orkambi, Symkevi and lastly Kaftrio, introduced in 2021.
Epidemiology and Treatment development
- Autosomal recessive disease
- Affecting 1:2500 people
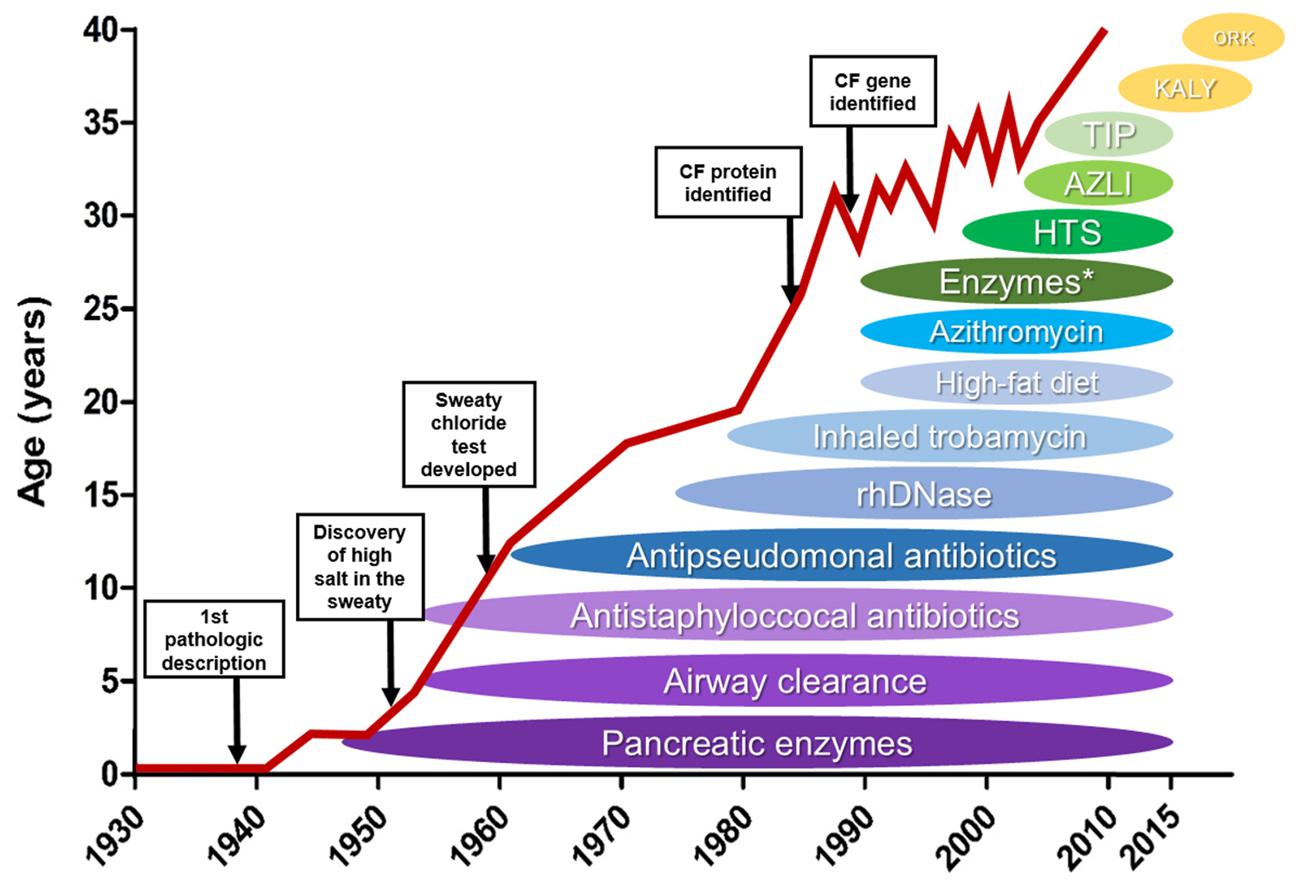
- Cystic Fibrosis was first described in 1935. At the time it was untreatable and deadly in childhood.
- Complications grow in severity with age and can occur in nearly every organ
- The treatments started with being focused on the symptoms: pancreatic enzymes to prevent malnutrition to treating the underlying cause - defective CFTR gene
- This lead to an increase in life expectancy over time (see graph)
CFTR Gene and Modulators
- In 1989 the CF transmembrane conductance regulator (CFTR) gene was discovered - responsible for production of viscous and sticky mucous
- With the introduction of newborn screening life expectancy has risen to approximately 40 years.
- 8 classes of CFTR mutations were detected, affecting different functions of the cells in the lungs
- Most common mutation: Phe508del mutation which belongs to the classes II, III, IV
- There are 4 CFTR agents currently available in the UK - Kalydeco (2016), Orkambi (2019), Symkevi (2020) and Kaftrio (2021) which are aimed at the population with the Phe508del mutation and other common ones (90% of CF population)
Methodology
- Medline, PubMed and EMBASE, three databases were searched: "cystic fibrosis" OR “CF”, “CFTR modulators” OR “ivacaftor” OR “tezacaftor” OR “lumacaftor” OR “elexacaftor”, “efficacy” OR “safety"
- Inclusion criteria: 1. Randomised control trial, 2. Measuring primary and secondary criteria (ppFEV1, PEX, chloride sweat, BMI and adverse effects) and Full-Text Study
- Exclusion criteria: Written in another language than English and not peer reviewed
- Number of journals identified: 99, identified as relevant: 37, included in this review: 23
Findings
(Forced Expiratory Volume in 1 Second)
FEV1
FEV1 is a diagnostic tool assessing lung function. This was conducted in patients <12 years old. (Listed from top (most effective) to bottom (least effective))
- Kaftrio: +13.8% from baseline and reduced PEx by 63%
- Kalydeco: +10.6% from baseline and reduced PEx by 53%
- Symkevi: +6.85% but Orkambi had no significant findings
- PEx reduced by 30-35%, however if administered to non-Phe508del mutation patients, the results were insignificant
Adverse Events
(AE's)
Overall well tolerated.
Orkambi showed the most significant side effects with shortness of breath, cough and pulmonary exacerbations. Discontinuation rate: 4.2%
Sweat Chloride and Body Mass Index
(SwCl and BMI)
Used as a diagnostic tool and measures overall reduction. BMI measured as weight gain for CF patients is difficult. Youngest children had the best outcome overall.
- Kalydeco in adults: -48.1 mmol/L and in children: -73.5 mmol/L, BMI: +0.8 kg/m2
- Kaftrio: -39.1 mmol/L, BMI: 1.03 kg/m2
- Symkevi: only -6.04 mmol/L but not significant in BMI
- Orkambi: not significant in either
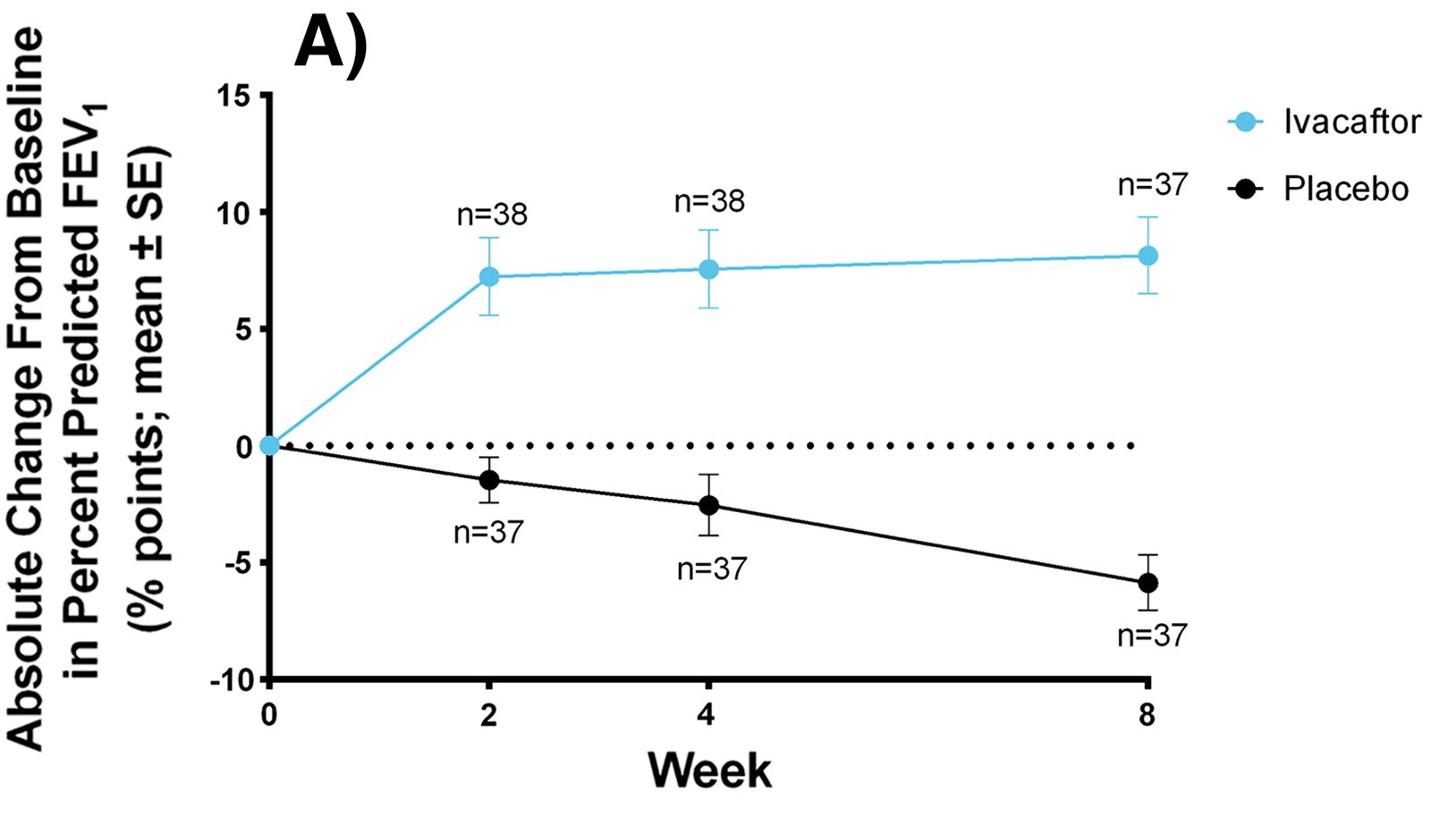
Graph showing improvement of FEV1 using Kalydeco over the span of 8 weeks
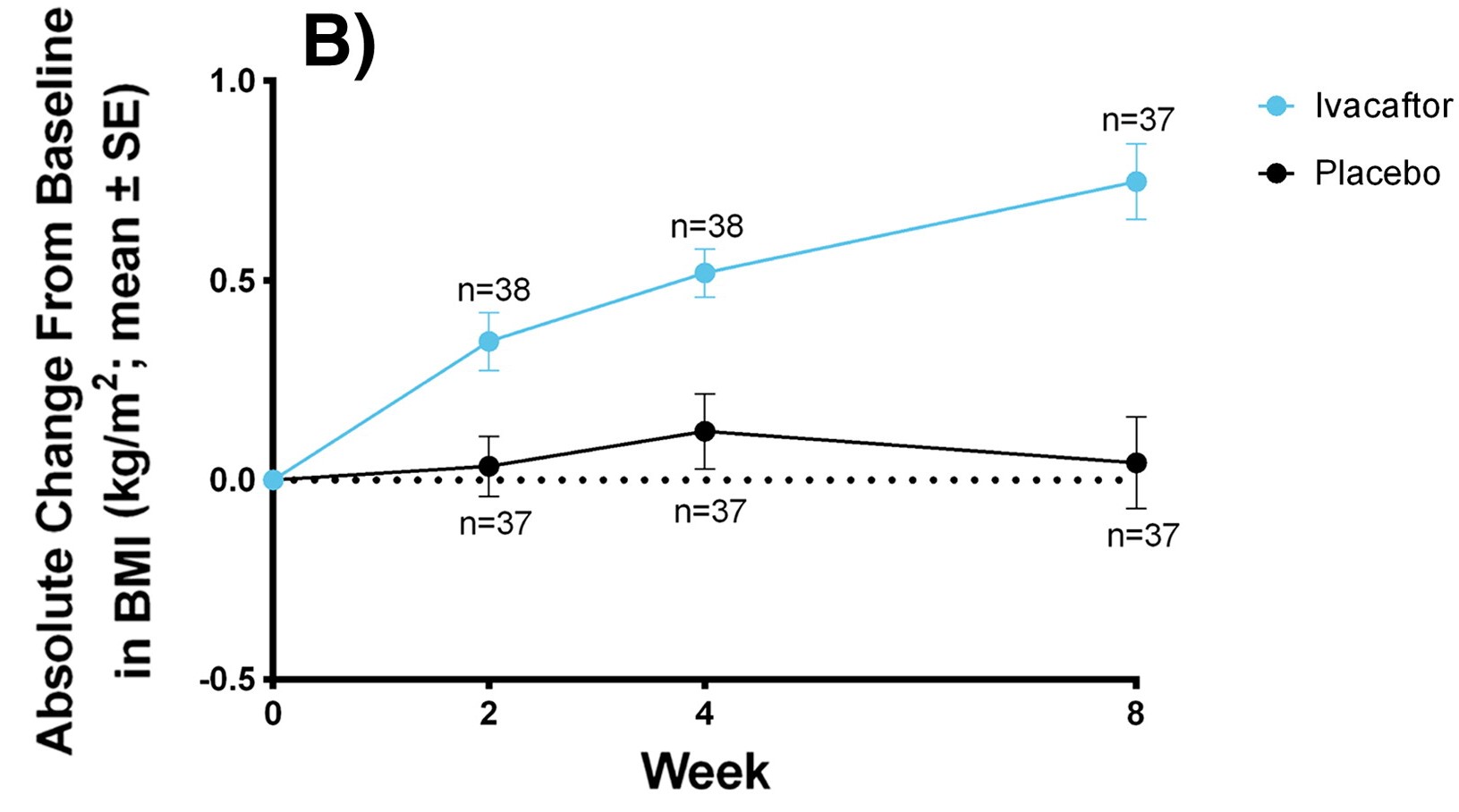
Graph showing improvement of SwCl using Kalydeco over the span of 8 weeks
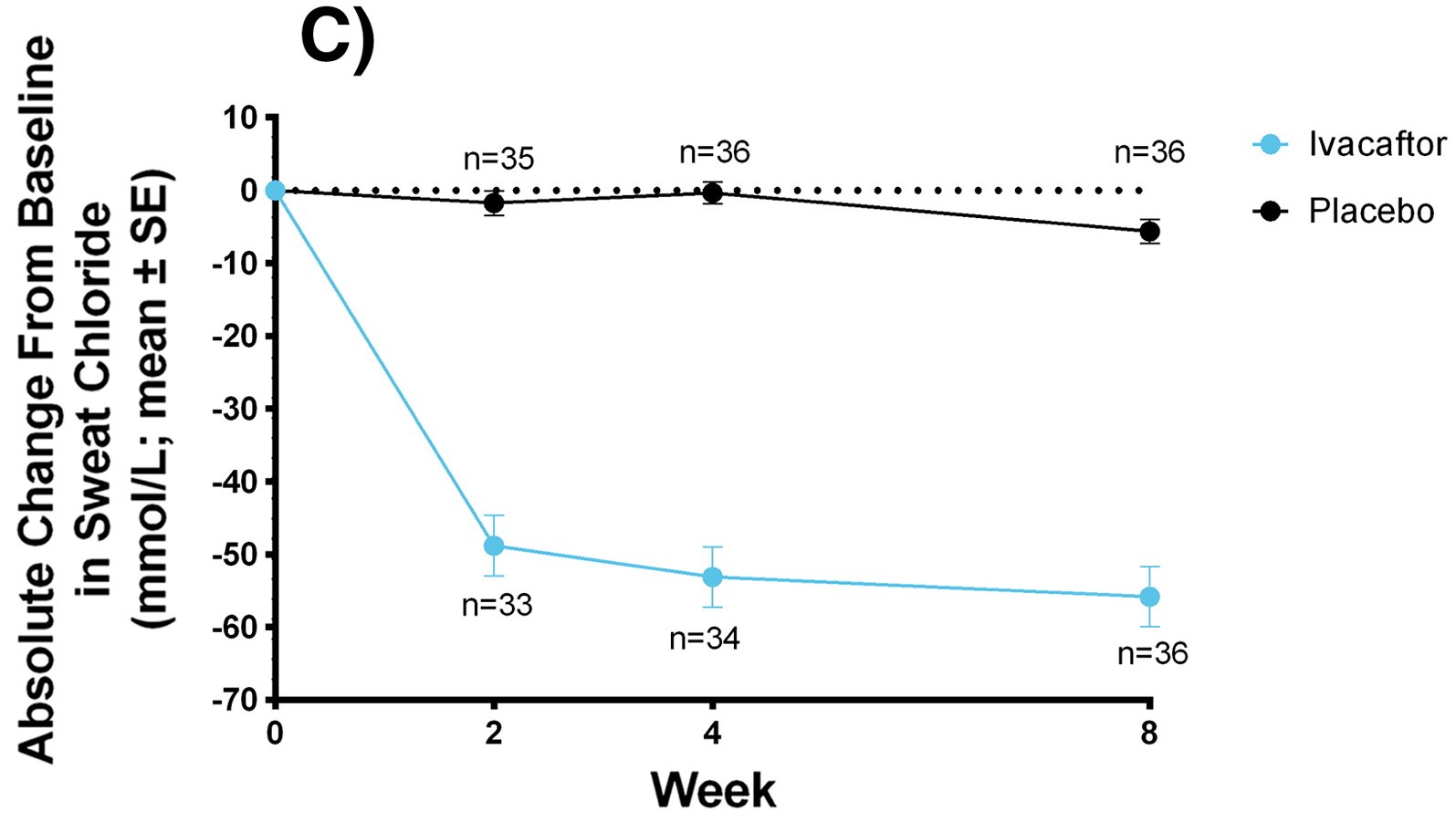
Graph showing improvement of BMI using Kalydeco over the span of 8 weeks
Dicussion
Best primary outcomes seen in patients with common mutations (e.g. Phe508del) receiving Kaftrio and Kalydeco. Lung function (FEV1 and PEx) improved as well as the patients quality of life. The younger population (12 months - 6 years) experienced the greatest improvements.
Orkambi and Symkevi were exclusively effective in Phe508del mutation, limiting the population size and the overall effectiveness.
Even though the CFTR modulators were well tolerated, for children <6 years old, Kalydeco is the only agent approved to be used. Nevertheless, this significantly prevents lung deterioration and causes a significant improvement in their quality of life.
A simulation model predicted, that the earlier treatment (Orkambi) is initiated, the median survival rate increases accordingly (up to +23.4 years). Early treatment can prevent lung function deterioration and consequently fatal future outcomes. This can be applied to all CFTR agents.
10% of the CF population carry different and more unique mutations which the CFTR modulators are not effective for, hence limiting the treatment options for those patients drastically. Research is continuing to expand and new possibilities are being explored, for example gene editing.
References
- Cystic fibrosis symptoms and treatments [Internet]. Nhsinform.scot. 2021 [cited 8 December 2021]. Available from: https://www.nhsinform.scot/illnesses-and-conditions/lungs-and-airways/cystic-fibrosis#treating-cystic-fibrosis
- De Boeck, K., 2020. Cystic fibrosis in the year 2020: A disease with a new face. Acta Paediatrica, 2020;109(5), pp.893-899
- Pettit RS, Fellner C. CFTR Modulators for the Treatment of Cystic Fibrosis. P T. 2014;39(7):500-11
- Gramegna, A., Contarini, M., Aliberti, S., Casciaro, R., Blasi, F. and Castellani, C. From Ivacaftor to Triple Combination: A Systematic Review of Efficacy and Safety of CFTR Modulators in People with Cystic Fibrosis. International Journal of Molecular Sciences, 2020;21(16), p.5882
- Dagenais R, Su V, Quon B. Real-World Safety of CFTR Modulators in the Treatment of Cystic Fibrosis: A Systematic Review. Journal of Clinical Medicine. 2020;10(1):23.
- Lopes-Pacheco, M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Frontiers in Pharmacology, 2020;10:1662
- Habib, A., Kajbafzadeh, M. et al. A Systematic Review of the Clinical Efficacy and Safety of CFTR Modulators in Cystic Fibrosis. Scientific Reports, 2019;9(1):7234
- Bierlaagh MC, Muilwijk D, Beekman JM, van der Ent CK. A new era for people with cystic fibrosis. Eur J Pediatr. 2021;180(9):2731-9.
- Gavioli, E., Guardado, N., Haniff, F., Deiab, N. and Vider, E., A current review of the safety of cystic fibrosis transmembrane conductance regulator modulators. Journal of Clinical Pharmacy and Therapeutics., 2020;46(2):286-294.
- Kalydeco [Internet]. CF Trust. 2021 [cited 8 December 2021]. Available from: https://www.cysticfibrosis.org.uk/the-work-we-do/campaigning-hard/life-saving-drugs/kalydeco
- Orkambi [Internet]. CF Trust. 2021 [cited 8 December 2021]. Available from: https://www.cysticfibrosis.org.uk/the-work-we-do/campaigning-hard/life-saving-drugs/orkambi
- Symkevi [Internet]. CF Trust. 2021 [cited 8 December 2021]. Available from: https://www.cysticfibrosis.org.uk/the-work-we-do/campaigning-hard/life-saving-drugs/symkevi
- FDA Approves Trikafta for Children Ages 6 Through 11 With Certain Mutations [Internet]. Cystic Fibrosis Foundation. 2021 [cited 8 December 2021]. Available from: https://www.cff.org/node/631
- Triple combination therapy Kaftrio (Trikafta in the US) [Internet]. CF Trust. 2021 [cited 8 December 2021]. Available from: https://www.cysticfibrosis.org.uk/the-work-we-do/campaigning-hard/life-saving-drugs/triple-combination-therapy
- Ramsey, B., Davies, J. et al. A CFTR Potentiator in Patients with Cystic Fibrosis and theG551DMutation. New England Journal of Medicine; 2011;365(18):1663-1672
Slide 1 image (max 2mb)
Slide 1 video (YouTube/Vimeo embed code)
Image 1 Caption
Slide 2 image (max 2mb)
Slide 2 video (YouTube/Vimeo embed code)
Image 2 Caption
Slide 3 image (max 2mb)
Slide 3 video (YouTube/Vimeo embed code)
Image 3 Caption
Slide 4 image (max 2mb)
Slide 4 video (YouTube/Vimeo embed code)
Image 4 Caption
Slide 5 image (max 2mb)
Slide 5 video (YouTube/Vimeo embed code)
Image 5 Caption
Slide 6 image (max 2mb)
Slide 6 video (YouTube/Vimeo embed code)
Image 6 Caption
Slide 7 image (max 2mb)
Slide 7 video (YouTube/Vimeo embed code)
Image 7 Caption
Slide 8 image (max 2mb)
Slide 8 video (YouTube/Vimeo embed code)
Image 8 Caption
Slide 9 image (max 2mb)
Slide 9 video (YouTube/Vimeo embed code)
Image 9 Caption
Slide 10 image (max 2mb)
Slide 20 video (YouTube/Vimeo embed code)
Image 10 Caption
Caption font
Text
Image (max size: 2mb)
Or drag a symbol into the upload area
















Image description/alt-tag
Image caption
Image link
Rollover Image (max size: 2mb)
Or drag a symbol into the upload area
















Border colour
Rotate
Skew (x-axis)
Skew (y-axis)
Image (max size: 2mb)
Or drag a symbol into the upload area
















Image description/alt-tag
Image caption
Image link
Rollover Image (max size: 2mb)
Or drag a symbol into the upload area
















Border colour
Rotate
Skew (x-axis)
Skew (y-axis)
Image (max size: 2mb)
Or drag a symbol into the upload area
















Image description/alt-tag
Image caption
Image link
Rollover Image (max size: 2mb)
Or drag a symbol into the upload area
















Border colour
Rotate
Skew (x-axis)
Skew (y-axis)
Image (max size: 2mb)
Or drag a symbol into the upload area
















Image description/alt-tag
Image caption
Image link
Rollover Image (max size: 2mb)
Or drag a symbol into the upload area
















Border colour
Rotate
Skew (x-axis)
Skew (y-axis)
Image (max size: 2mb)
Or drag a symbol into the upload area
















Image description/alt-tag
Image caption
Image link
Rollover Image (max size: 2mb)
Or drag a symbol into the upload area
















Border colour
Rotate
Skew (x-axis)
Skew (y-axis)