Meet Sophie: Quantum nanotechnology, building materials from the bottom up: is it worth the extra energy?

Hi, I am Sophie, a first year PhD student in Material Social Futures: a Leverhulme Doctoral Scholarship programme at Lancaster University. Previously I obtained a Bachelors and Masters in Chemistry, also from Lancaster. I was drawn to the MSF programme because of the unique opportunity to combine a scientific training with big picture thinking in sustainability.
I hope to be able to use knowledge and skills acquired in the MSF programme to pursue a research career that has the potential to shape a better future.
Thinking about energy:
Electrical energy is the most versatile form of energy that we can manipulate i.e., it can be converted into other “less noble forms” such as kinetic (mechanical), light, and various subdivisions. Conversion will always result in a loss of energy, due to the first law of thermodynamics. Ultimately, all energy eventually turns (or is indeed lost) into heat, causing us to regard heat as the lowest “quality” form of energy; despite this it represents more than 90% of energy usage.
How then can we recover this “lost” useful energy? The thermoelectric effect (TE) is one such phenomenon that has application in this quest. Thermoelectric materials can directly convert heat to electricity and have gained widespread attention for their potential applications as mini-cooling systems, TE generators, and self-powered sensors. Research into thermoelectric materials focusses on improving the efficiency of such materials, and developing materials that are cheap and industrially viable.
The relatively low price of energy is such that sustainability in terms of energy has not featured in societal thinking until recently. Yet, the energies used creating the components of a mobile phone are very large. For example, the CPU is mostly silicon and the process of extracting pure silicon from silicon dioxide involves heating at high temperatures of around 2200°C with carbon. This takes 1000-1500 MJ/kg. The energy used in this process is known as the embodied energy and is high (cf. aluminium = ca. 170 MJ/kg). The calculation of embodied energy can reach much further back into the manufacturing process, e.g. what about considering the energy used in getting the raw materials and energy to the factory in the first place? One of the complications of calculating embodied energy is how far back should you go?
The mobile phone and energy:
Normal working mobile phones operate between 25-30°C, but handsets can get up to ~50°C and if some of this waste heat were to be converted back into electricity, would our phones require less power from charging? (Although if we were to apply Jevons paradox – commonly seen in battery development – we can expect, rather than a reduction in energy use, an increase in the capabilities of the phone means no energy is saved at all!)
In terms of electrical energy recovery from waste heat, the question I am addressing in my PhD is whether enough energy is recovered to account for the further, additional embodied energy by the incorporation of a thermoelectric material within the mobile phone.
The efficiency η of converting heat into electricity is predicted from Equation 1.
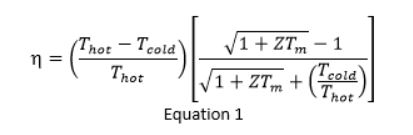
ZT is the figure of merit (a primary measure) of a material’s performance and helps in finding the efficiency. In most thermoelectric materials, ZT values are between 1 and 2.5.
If we take the “hot” temperature of the mobile phone to be 30°C, and the “cold” to be approximately just below room temperature at 25°C (and a ZT=2) the equation simplifies to:
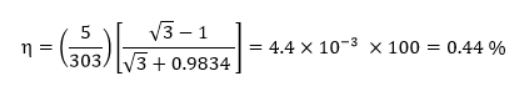
This provides a starting point to obtain a value for energy return on energy invested (ERoEI) - at least for phone charging!
_________________________________________________________________________________________________
A simple calculation:
If assuming that the charging efficiency of a typical phone battery is 80%, and an iPhone XS has 6.96 Watt hours.
6.96 Watt hours = 25056 Joules so 20% of waste heat = 5011.2 J
0.44% of this is 22.05 Joules.
With a 2 year contract and charging every day = 730 x 22.05 = 16095.9 J.
There are estimated 2.7B mobile phone users worldwide. Therefore, potential for 43T J saving per 2 years.
This sounds like a lot, but to put it into context, what does this means for a single phone?
To power a 16 Watt LED light bulb for 1 minute = 960 Joules.
So, using a thermoelectric coating you could power a light bulb for 16.7 hours with 2year savings on one phone.
Alternatively, assuming we achieve an 80% charge (20044.8 J), after 909 recharges we get one charge for free (which does not seem a lot). Further, we need to consider ERoEI, and only then can we come up with a sensible target efficiency.
________________________________________________________________________________________________
The next challenge is to make an estimate of the embodied energy of a thermoelectric coating material. And this is the Material Social Futures component of my PhD.
I will develop a novel bottom-up technology to fabricate thin-film coatings for TE materials based upon bespoke organic compounds deposited on gold (imines deposition on gold). The challenge is to maximise ZT, such that the ERoEI accounts for the energy in molecule synthesis and purification, and for the energy used in making a gold coating (e.g. for maintaining ultra-high vacuum and the molecule sublimation).
Extending to the Big Picture:
When thinking about sustainability, I am appreciating that it goes beyond just the use of materials, and in the ‘big picture’, it is important to think about the value of the material compared to the effort put into making it (i.e. the embodied energy in a finished product vs. any gain in efficiency).
But, there are further big picture challenges: for example, where does the waste go? Consumers change phones every few years. Many electronic devices end up in municipal dumps. Eventually the thermoelectric material itself may decompose and potentially release toxic particles either harmful to humans, wildlife or the environment.
And through a ‘big picture’ approach, a priori it is possible to account for at least some the factors that should be addressed upfront in terms of the implications for society and the environment during and after manufacture of electronic consumer goods
In this context, I am starting to see ways by which it may be possible to steer to a future we would rather have than one we are “given”.
Back to News
