All Health and Social care research falling under the remit of the Secretary of State for Health requires regulatory approval(s) and a formal sponsor as set out in the UK Policy Framework for Health and Social Care Research. This UK policy framework has been developed with the Health Research Authority (HRA) and includes all research in health and social care that involves NHS patients, their tissue or information, or is funded with public funds. There are similar requirements for research involving social care practitioners, clients and resources, where this falls under the Secretary of State for Health’s remit.
Post-Award Phase
Identifying the Applications You are Required to Make
To determine if your work falls under the remit of the UK Policy Framework, you should first check if your project is defined as research by the HRA. A decision tool 'Is my study research' is provided on their website alongside other useful guidance. MSc and PhD students in a health related discipline and their supervisors can access a decision tool and guidance tailored specifically to them using the student research toolkit.
If the relevant decision tool indicates your work constitutes research under the UK Policy Framework you next need to refer to the guidance section on the HRA website 'My project is categorised as research' to determine if it will require HRA approval.
If your work is classed as research by the HRA, you will also need to use the decision tool 'Do I need NHS ethics review' to establish if you need NHS REC review and favourable opinion. Both HRA approval and NHS REC review and favourable opinion are applied for using the Integrated Research Application System (IRAS). If you do not need NHS REC review and favourable opinion, you may need to apply for Faculty REC approval.
Faculty REC approval will be required where your project is classed as research, requires HRA approval but not NHS REC review and favourable opinion. Faculty REC approval might be required in the instance where your project is not classed as research, or classed as research but does not need HRA approval or NHS REC review and favourable opinion. For faculty REC approval you will need to apply for this using REAMS.
It is important to realise all research requiring HRA approval and/or NHS REC review and favourable opinion requires a sponsor. Lancaster University will usually sponsor research when an employee has designed the study and is acting as the chief investigator or where the project is being conducted by a Lancaster University student in order to obtain an educational qualification. In the instance where your project requires or has been granted NHS REC favourable opinion, but your sponsor is external, you may also still need Faculty REC. If you are unsure who to approach to act as your sponsor, you can contact us for more support.
The PDF below contains a flow chart to help identify what approvals, ethics and sponsorship your research might need, and the subsequent applications you will be required to make.
Identifying Approvals Ethics Sponsorship and Subsequent Applications PDFMaking Your Applications
Below is guidance on which applications you will need to draft and submit, based on what approvals, ethics or sponsorship you require.
Faculty REC – REAMS application
If you need Faculty REC approval you will need to apply for this using REAMS. Please see the Research Integrity, Ethics & Governance - Lancaster University site for guidance on this process.
Please note if you are applying for Lancaster University sponsorship as well, you should apply for sponsorship before you apply for your Faculty REC approval. As you will be required to provide confirmation that a sponsor is happy to support your work before obtaining a REC opinion. In this instance, you do not need a draft REAMs application to apply for sponsorship.
HRA Approval and NHS REC Review and Favourable Opinion – IRAS application (Integrated Research Application System)
To apply for HRA approval and/or NHS REC review and favourable opinion you will need to use the Integrated Research Application System (IRAS) to complete an IRAS application form. One form can be used to seek approval for both. The below accordion provides general as well as Lancaster University specific guidance for completing your IRAS form.
To request Lancaster University sponsorship, you will need to create a draft of your IRAS form, download this as a PDF before the submission page point (see Guidance 'How to Save and Export IRAS as a PDF), and send this to us with sponsorship request form (see Lancaster University Sponsorship section). Please note, once sponsorship has been approved you will then need to submit your IRAS (see Guidance 'How to for IRAS submission').
Accordion for IRAS application. accordion
Guidance and training for completing your IRAS form is available in detail from the HRA. Their webpages have comprehensive information about gaining approvals. The IRAS website also has information on how to prepare and submit an application for HRA and HCRW Approval and the 'i' buttons on IRAS have helpful information to help you answer each question correctly.
The IRAS form is an intuitive form, that opens the required forms and questions based on your selections on the project filters. Please ensure your filter questions are completed correctly to open the correct questions. No questions should be left blank on your IRAS form. Instead, please indicate the question is not applicable by adding 'n/a'. Leaving questions blank will invalidate the sponsor signature and could cause delays for your project.
If you have any technical difficulties in IRAS you can contact the IRAS Technical Helpdesk on 0207 043 0734 or email them at helpdesk@myresearchproject.org.uk. For other queries, contact HRA queries. As the IRAS form is not part of university processes, we are only able to offer limited guidance regarding technical aspects of the online system.
Please refer to the below Lancaster University specific guidance for completing your IRAS form which should help your application progress smoothly. If you are a student, remember to seek additional advice from your supervisor.
These notes should help you complete the IRAS form:
- Clinical trials/investigation– please bear in mind that this has a very specific meaning within the NHS. If you are unsure whether your study may be a clinical trial or investigation please contact us.
- Student applicants-Please answer 'yes' to question 9, 'Is the study or any part of it being undertaken as an educational project?'. This includes PhD projects and those being undertaken as part of an educational or research qualification as continuing professional development (CPD).
- Student applicants: Sections A2-2 & A3-1 -HRA guidance notes that students should not normally take the role of chief investigator. Exception is made for an experienced care practitioner or manager undertaking an educational qualification for continuing professional development, or for an experienced healthcare professional undertaking a doctoral-level study who is in receipt of a fellowship. Please discuss this with your supervisor before completing these sections.
- Sections A4 & A64 -The contact on behalf of the sponsor is Becky Gordon, Head of Research Quality and Policy, Lancaster University, LA1 4YT, email sponsorship@lancaster.ac.uk.
- Section A14-1 -This section is asking about involvement of patients, services users, carers or the public. This is referring to PPI that has taken place. This should be done ahead of your submission to sponsorship and the information obtained should influence your project design and materials. PPI review of your protocol and participant facing documents is mandatory as of 1st December 2023, even if this is just with a single individual with lived experience or stakeholders from the community you are studying.
- Section A22- It is appropriate to mention the process for participants withdrawing from the research in this section.
- Section A23- If this section is answered with a 'yes', then the sponsor requests that you provide a distress protocol with your submission, outlining the process for how you will deal with distress (even minor distress).
- Section A26- Include details here of the Lancaster University Lone Worker/Researcher Policy, outlining the main elements of it that apply to your project.
- Section A27-1- Here you should ensure that only members of the direct care team at your research site access personal information before written consent has been provided, to identify or approach participants. If you want to access personal information without consent, outside of the direct care team, it is not advised, although there are methods of obtaining permissions to do so, as a last resort (see CAG information).
- Section A37- State that data on portable devices will be encrypted; if it cannot be encrypted, confirm that any identifiable data (including recordings of the participants' voices) will be deleted from the recorder as quickly as possible (e.g. when it has been transferred to a secure medium, such as Lancaster University approved secure cloud servers (one driver)) and in the meantime the recorder will be stored securely in a locked cabinet on University or NHS premises.
- Section A51– It is important that you tick all reports and dissemination methods that apply.
- Section A68-1- All studies that require HRA approval need to have a lead NHS R&D listed. This should be discussed with an NHS R&D or R&I department to see if they will act as such. The lead NHS R&D are to act as an NHS point of contact for support and guidance on NHS specific issues. The contact listed must be someone who is able to authorise acting as such, on behalf of the R&D department. Usually, one of your research sites or a site you have a relationship with can act as such, but this should be discussed with them ahead of submitting your IRAS and it does not obligate them to be a research site.
- Section A76 – 1,2,3 -Please read the notes in the insurance section(s) on the IRAS form: if Lancaster University’s insurance cover applies insert the phrase ‘Lancaster University legal liability cover will apply’ in the insurance section(s) as appropriate and ensure that you append the Lancaster University insurance documents to your application. Should you be making use of the NHS Indemnity Schemes, please make this selection and enter 'NHS Indemnity Scheme will apply'.
It is the researcher’s responsibility to ensure they are covered by the University research policy. Some types of research require additional cover to be purchased. Please contact the insurance department to see if you are covered under our clinical trials policy, or if you may need to purchase additional cover from your grant budget. The clinical research team will also consider insurance cover during the review but may refer you to the insurance team to explore this in more detail.
- Section A78 -Could the research lead to the development of a new product/process or the generation of intellectual property? We recommend that you select ‘Yes’ if:
-
- your research could lead to the development of a new product/process
- you are producing anything other than scholarly works*.
*Definition of Scholarly works: “Scholarly works shall include [but not be limited to] Journal Articles, Conference Papers, Theses and Essays, Textbooks”.
To note, if you wish to change your answer to this question after your project has been approved you can submit an amendment (See step 11).
- Section D2 -Please do not complete the ‘declaration by the sponsor's representative’ section (this must be completed by the University after sponsorship has been confirmed in writing). The signature from your CI and your supervisor should be requested post sponsorship, however, they should still have read your documents ahead of submission to sponsorship.
- Section D2-Please ensure that you have completed the validation of your application ahead of requesting e-signatures from the sponsor and CI, as any changes made after this point will invalidate signatures.
Lancaster University Sponsorship – Internal sponsorship application
If you would like to name Lancaster University as your sponsor, you will need to follow the guidance on this page to request sponsorship. Upon receiving your request, the Clinical Research Governance team, in collaboration with the Health and Social Care Research Sponsorship Committee, will undertake a review of your application, and work with you to submit the required paperwork to the regulators. Once we have ensured your application is complete, compliant and the risk is acceptable, we will confirm full sponsorship (where possible) and insurance cover. Please allow up to 30 days for us to process your application and to confirm if we can sponsor your study. Details of our review process can be found in our SOP located here - Standard Operating Procedures and summarised within a flow chart located here - Guidance.
Lancaster University requires you to complete the following documents to request sponsorship. Items marked with an * are mandatory for all sponsorship requests, whilst items marked with ** are mandatory in a project specific manner. Detailed guidance on completion of each document can be found by opening up the accordion below.
Please consult these materials and guidance notes when compiling your application as errors in applications can cause delays. During the process we will reach out to you with queries and amendments that will need actioning from you. Delays hearing back from you will impact the overall timeline to potential sponsorship confirmation.
Accordion for Sponsorship application. accordion
Please ensure the information provided is consistent with the details on your IRAS form. Ensure you fill out the correct version numbers and dates for the documents you send to us.
This must be provided using the qualitative HRA template. State any aspects that are non-applicable and remove all guidance wording before submission. Protocol guidance.
Please note the definition of the end of a study must documented in the study protocol (this should be descriptive rather than a date or timeframe). For most clinical research the definition will be "following the last visit of the last participant"; or "following the completion of any follow-up monitoring and data collection".
Protocols for studies clinical trials or interventional health and social care studies (which fall within the first four categories in IRAS) must be registered before the first participant is recruited and no later than six weeks after. This is mandatory to obtain favourable NHS REC opinion (research registration guidance) and is a requirement for publication in a growing number of journals (International Committee of Medical Journal Editors (ICMJE)). For all other study types, it is considered best practice for the protocol to be published.
Lancaster University recommends using one of the following registers:
- International Standard Randomised Controlled Trial Number Register (ISRCTN). There may be a charge for registration. Some funding bodies require ISRCTN registration.
- EudraCT is the European clinical trials database of all clinical trials. Registration is compulsory for every clinical trial with at least one site in the European community, and provides a unique identification number for the trial.
The form can be found here - Forms. Complete all the relevant sections, if a section is not-applicable please select NA. You should ensure you use the scoring matrix to calculate a score, and put in place appropriate mitigations based on the initial risk score for each factor. For more information please refer to our SOP which can be found here - Standard Operating Procedures.
As well as the Sponsorship Risk Assessment, you should check if a health and safety risk assessment is also required. When you submit your application to the sponsorship team, please state whether a university health and safety risk assessment has been carried out, and if not please confirm the reason.
To note, carrying out health and safety risk assessments fall under the remit of the Safety Office. If you are a staff member, please contact your Area Safety Officer for guidance, alternatively students should approach their supervisors.
Management of data is an essential part of good research practice and all researchers in the University have an obligation to record, store, share and archive their data appropriately. Most research funders and many academic publishers now have mandates that require research data to be properly managed.
All staff and students involved in collecting or handling research data are required to create a Data Management Plan (DMP) on the appropriate template. Relevant funders templates take precedence, please see the funder expectations pages for more information. Where a funders template is not specified, then Lancaster University’s template must be used. Please refer to the Library's research data management webpage for more information, guidance and training. We draw you attention particularly to the Research Data Sharing and Archiving Policy and the Research Data Policy when generating your data management plan. Where the Lancaster University’s template is being used you might find it helpful to use the Digital Curation Centre’s DMPonline Tool to generate your DMP as it contains step-by-step instruction. Researchers are strongly encouraged to publish the DMP openly (using services such as Zenodo), or it can be shared easily within the Digital Curation Centre’s DMPonline Tool.
Please ensure your data management plan is consistent with the data arrangement information you provide within your protocol, IRAS application, consent procedure and participant information sheets. You should also ensure that the initial consent and PIS covers the ways in which you may use data.
Please be aware, that for those working with the NHS or with NHS data, you may be asked about ISO certification; the University is not certified in the ISO27001 family of standards, but adheres to the principles of them, which meets all Research Data Management requirements.
The information held within the data management plan is needed to show legal and ethical compliance with the Data Protection Act and General Data Protection Regulations (GDPR) that may be required to deliver your research in a health and social care setting. It is also needed to identify the need for data sharing and data processing in contracts and agreements. Please see accordion section on contracts and agreements.
- NHS Research Site Agreements
An NHS site agreement is needed to ‘sub-contract’ the NHS site to support data collection, and agree terms such as payment, resources, data sharing, data processing and material transfer. Below are details of the three most common non-commercial NHS site agreements required by Lancaster University researchers. The agreement template you need to draft for submission will depend on the type of site you are setting up and your study type. If you need support in deciding which agreement you require, or completing them, please contact us for more guidance. More information from our research contracts team is available on the Clinical Research Contracts Guidance (internal link) section of the Research Services website.
1. Organisation Information Document (OID) - For non-interventional studies that are classed as full research sites (activities in addition to participant identification and handing out Patient Information Sheets happen on site even if this is by the researcher.)
Here is the link to the OID guidance and the template.
Please also ensure that the following wording is used for question 14:
'The Sponsor expects that researchers and local site staff complete any training relevant to the proposed area of research (e.g. any relevant clinical training, or training relating to the use of specific equipment/devices/questionnaires) before research commences. This includes Good Clinical Practice Training renewed every 3 years for non-interventional work and every 2 years for interventional work.'
2. Model Non-Commercial Patient Identification Centre Agreement (mNC-PICA)- for non-interventional studies that are participant identification centres only, where the sponsor is contracting directly with the PIC site, a model Non-Commercial PIC agreement (mNC-PICA sponsor to PIC) should be used. Participant Identification Centres (PICs) are NHS/HSC organisations that identify potential research participants and no further activity or involvement from the site takes place. Please note that an SoE is not required to be submitted with this type of agreement see next section on Schedule of Events or Schedule of Events Cost Attribution Template.
Here is the link to PIC information.
Here is the link to the mNC-PICA sponsor to PIC guidance and template.
3. UK-wide model Non-Commercial Agreement (mNCA) – for interventional research scenarios.
Here is the link to the mNCA guidance and template.
- Schedule of Events or Schedule of Events Cost Attribution Template
Schedule of Events (SoE) or Schedule of Events Cost Attribution Template (SoECAT) is required for all non-commercial studies. This will help to ensure that the appropriate resources are identified to support study delivery, and that there is clarity for participating NHS/HSC organisations about how the costs associated with participating in a study are attributed by itemising the activities taking place on site and allocating costs to the relevant funding sources.
If a SoECAT has been completed as part of the funding application, this should be provided, if not a SoE should be completed. Even if there is no funding associated with your research, a SoE is still required to itemise activities.
Each SoE or SoECAT should be accompanied by a site agreement. The only exception is if you are setting up Participant Identification Centre (PIC) sites which do not require a SoE.
Please note that SoE should be accompanied by an agreement, so if multiple SoEs need to be completed to cover site-level activities, multiple agreements must accompany them. This might occur for instance if you have a group of sites undertaking the same activities and other groups undertaking different activities to the first group.
Here is the link to SoE / SoECAT guidance and template.
If you need further support please contact us for more guidance.
- Data Processing or Transfer Agreements
If you have determined that your research involves processing or requesting another institution or person process personal data on your behalf, and/or you are sharing personal data or requesting that another party shares personal data with you, you may require an agreement or contract to cover the particulars.
Where this is the case, the researcher should contact the RSO Contracts team on rso-contracts@lancaster.ac.uk with detailed information about the proposed sharing arrangements, so an agreement can be drafted.
- Collaboration agreements
If your research involves collaboration between multiple institutions or parties you will need a collaboration agreement. In this instance please contact RSO contracts on rso-contracts@lancaster.ac.uk.
- Other Contracts and Agreements
If none of these contracts or agreements meet your needs please contact RSO contracts on rso-contracts@lancaster.ac.uk and visit Research Services website for more information.
Please attach your GCP certificate, it is a requirement that all researchers must complete the NIHR Good Clinical Practice (GCP) training online before applying for sponsorship. You can find out more about GCP training and how to sign up to the e-learning course on the NIHR webpage.
This does not need to be a full academic CV, 2 pages is sufficient, please see investigators CV template.
Researchers hold the responsibility to ensure their research is covered by the University's insurance policies. In order to check if your research is covered by existing University insurance policies, refer to the guidance. Some types of research require additional cover to be purchased, and all additional insurance will need to be covered by the research grant.
Insurance checks should be carried out before you apply for your grant, or for unfunded research, you must check the guidance before committing to completing a project. If you need any additional help regarding insurance cover please contact the insurance department.
When applying for sponsorship please inform the clinical research governance team of the outcome of your insurance checks. This can be done using the dedicated space within the Sponsorship Request Form (HSCR-FORM002) and including evidence of your checks in your application. The clinical research team will consider insurance cover during the sponsorship application review process, and may refer you to the insurance team. Ensuring adequate insurance ahead of sponsorship application will avoid unnecessary delays during the review process.
If funding has been obtained please can you provide the confirmation of funding letter with your application.
Validated questionnaires need not be included but must be correctly referenced in the forms and version controlled. Any new questionnaires or unvalidated ones should be included for review. On all patient facing documents include the Lancaster University logo and leave a space marked as where the site logo can be added post approvals in addition to the collaborator/funder logo where relevant. Also include the version number, version date, document title and IRAS number. These details can be included using a header and a footer as depicted below. Those where the researcher will complete them themselves and not the participant, do not require logos, but do require the references to version number, version date, document title and IRAS number.
As these are not patient facing, please just include the version number, version date, document title and IRAS number in a suitable location, for example in a footer as depicted below.

Your Participant Information Sheet (PIS) should describe clearly what a potential participant should expect if they agreed to take part in your study. All faculties are to use and adapt the FHM Sample Participant Information Sheet template, scroll down to Documentation and Guidance 1 and the template can be found under Templates, examples and guidance.
Both the University and the HRA require specific wording to be given on your Participant Information Sheet, as well as project specific information on who will have access to personal data, where this data is held and how long for, and who the sponsor of the project is. The HRA website contains a wealth of information to create a PIS, and contains a link to their quality standards. Inclusion of these is mandatory, and all PIS documents submitted to the HRA or NHSREC must follow the quality standards.
As well as the template content, the HRA transparency wording must be included verbatim to explain how we use and manage personal data. This can be found on their website, under 'In the PIS or document provided to participants' section. Please ensure you only make edits to this section where prompted and add in information relevant to the study where prompted only.
Where the template wording asks for:
- The leaflet link, please use this webpage link: www.lancaster.ac.uk/research/data-protection
- Contact details, please use the DPO title and email address: Lancaster University Information Governance Manager, information-governance@lancaster.ac.uk
- Telephone, please use the main telephone number: +44 (0)1524 65201.
You will also be required to include a statement of insurance in your PIS, that informs participants what they can do if they are harmed during the study. Where only Lancaster University indemnity applies for the management, design or conduct of your study (as declared in your IRAS section A76-1 to 3) please include a header relating to 'What happens if I am harmed as a result of taking part?' section, and include the below statement:
‘Lancaster University holds appropriate indemnity cover which includes but is not limited to Public Liability, Professional Indemnity and Employers Liability Insurance. If you are harmed whilst taking part in this study as a result of negligence by Lancaster University or its staff members, you may have grounds for legal action and should obtain independent legal advice. Non-negligent harm is not covered, and any claims that arise may be referred to the insurance provider for assessment. Should you require more information on the indemnity cover that Lancaster University holds, please contact the researcher.‘
Where NHS indemnity has been selected instead of Lancaster University Indemnity, please contact your NHS lead site for their indemnity statement.
Where a mix of Lancaster University and NHS indemnity has been selected, please include the above Lancaster statement, and also include the below or contact your NHS sites to ask them to provide some additional wording to cover their indemnity:
'Where harm has occurred whilst taking part in this study as a result of negligence by the NHS, you may have grounds for legal action and should contact the NHS trust where you took part in the study for more information.'
On all patient facing documents include the Lancaster University logo and leave a space marked as where the site logo can be added post approvals in addition to the collaborator/funder logo where relevant. Also include the version number, version date, document title and IRAS number. These details can be included using a header and a footer as depicted below.
To record participants written informed consent, please ensure that all pertinent points are included, such as data arrangements, access to any medical records required, confirmation of consent for recording interviews. Please ensure that the consent form confirms only information from the PIS, as they are required to be informed before consenting to the statement. It is standard practice to collect initials rather than ticks next to each statement. You are also required to state what will happen with the consent form after the completion and who will retain copies. For example, “One copy to be shared with the participant and one copy to be retained by the research team.”
All faculties are to use the FHM Sample Consent Form template, scroll down to Documentation and Guidance 1 and the template can be found under Templates, examples and guidance.
The HRA website provides a wealth of information on informed consent, please see Informing participants and seeking consent - Health Research Authority.
On all patient facing documents include the Lancaster University logo and leave a space marked as where the site logo can be added post approvals in addition to the collaborator/funder logo where relevant. Also include the version number, version date, document title and IRAS number. These details can be included using a header and a footer as depicted below.
When referencing the relevant PIS as part of a consent statement, we recommend you leave a space marked for detailing the relevant PIS version on the date that consent is taken as the PIS often undergoes version edits. For example:

If it is indicated in the patient information sheet and protocol that contact with a GP/Health Professional may be required, a copy of the letter should be included. You should also remember to include the Lancaster University logo and leave a space marked as where the site logo can be added post approvals in addition to the collaborator/funder logo where relevant. Also include the version number, version date, document title and IRAS number. These details can be included using a header and a footer as depicted below, so that these details are included at the top and bottom of the GP/Health Professional Letter.
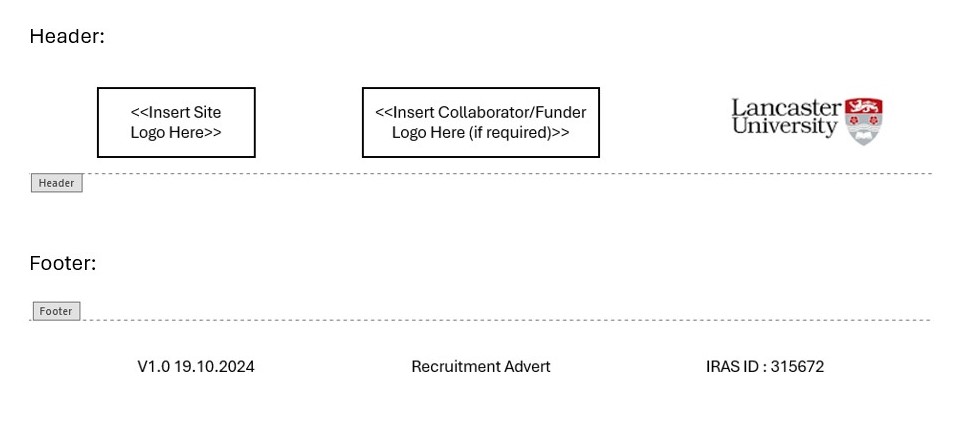
On your recruitment advert(s) remember to introduce yourself and detail that Lancaster University is sponsoring. On all patient facing documents include the Lancaster University logo and leave a space marked as where the site logo can be added post approvals in addition to the collaborator/funder logo where relevant. Also include the version number, version date, document title and IRAS number. These details can be included using a header and a footer as depicted below.
If peer review has been undertaken please can you provide the evidence with your application.
The delegation log provides clarity regarding who is responsible for undertaking what activity during delivery of the study at the NHS research site. The delegation log is a tool to be maintained throughout the lifetime of the study by each NHS research site. A delegation log is appropriate for studies that are clinical trials (defined as first four categories in IRAS Project Filter question 2) where the local study activities are undertaken at a NHS research site and managed by a local Principal Investigator (PI) on behalf of the Chief Investigator at Lancaster.
The IRAS website contains more information and a link to the template.
Note regarding student applications - undergraduate students are not currently eligible to apply to the HRA or NHS REC, but they can support on a health and social care research project (including the application for HRA approval, ethics and sponsorship) if this is led by a staff member such as a supervisor. MSc students are only eligible to apply to the HRA or NHS REC for their own project if they are undertaking a health-related course. Generally, PhD researchers should be named as ‘students’ and their supervisor or a University staff member should be listed as the CI. Only student researchers who are in receipt of a fellowship or undertaking an educational qualification for continuing professional development (CPD) should be named as their own CI. When students or supervisors use the student research toolkit, the final decision tree determines eligibility to apply for HRA approval and/or NHS REC review and favourable opinion based on the research eligibility criteria. The results of this must be appended to your IRAS form.

