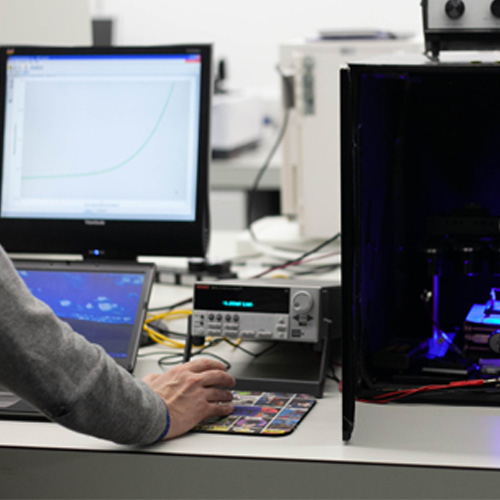
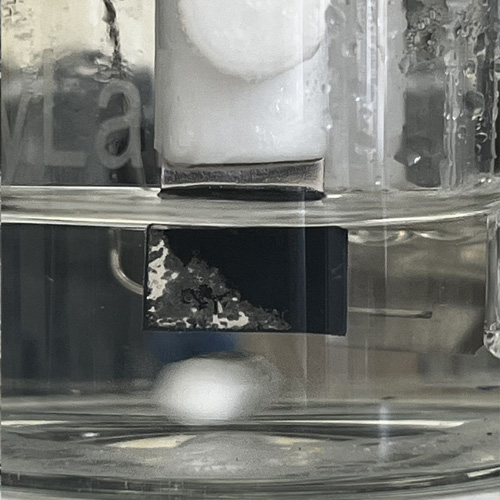
Researchers in the Chemistry at Lancaster are developing new chemistries to convert and store renewable energy, supporting the transition towards net-zero carbon emissions.
As a major part of our research, we are developing a diverse range of chemical solutions to meet the energy demands of 21st century society.